NEW
YORK (Reuters Health) - A multivalent HIV vaccine that consists of
envelope protein (gp120 Env) and either DNA or NYVAC vectors during
priming leads to early and potent IgG-binding antibody responses,
according to results from the HIV Vaccine Trials Network (HVTN).
"If the ongoing vaccine efficacy studies show significant levels of protection against acquisition of HIV, future vaccine studies may be designed to induce rapid appearance of antibody responses as shown in our study," Dr. Giuseppe Pantaleo from the Swiss Vaccine Research Institute, Lausanne University Hospital, told Reuters Health by email.
Dr. Pantaleo and colleagues in the HVTN 096 trial tested their hypothesis that coadministration of vaccines containing Env protein during priming in a heterologous prime and boost regimen would induce early generation of an antibody response against the conserved regions of the V1/V2 loop of Env, thereby extending the period of protection seen in an earlier trial (RV144).
Specifically, they compared vaccine regimens comprising coadministration during priming of either a DNA or NYVAC vector and gp120 Env protein versus DNA or NYVAC vector alone followed by a NYVAC vector and gp120 protein boost.
The trial enrolled 96 individuals at low risk of HIV
infection. Twenty participants were randomly allocated to each of four
treatment groups and four participants were assigned to each placebo
group.
Vaccines containing the NYVAC vector triggered more-frequent severe reactogenicity and more adverse events than did vaccines containing the DNA vector, the researchers report in The Lancet HIV, online October 7.
Co-administration of gp120 Env protein during priming resulted in earlier peak neutralizing antibody responses, compared with regimens lacking gp120 Env protein at prime.
Gp120 Env protein co-administration during priming also yielded a significantly higher overall neutralizing antibody response (as measured by AUC), compared with groups that did not receive gp120 Env protein co-administration at prime.
For all groups, antibody responses and magnitudes of response decreased between two weeks and 12 months after the month-six vaccination, and there were no differences in the aggregate vaccine-matched response magnitudes between groups at 12 months after the month-six vaccination.
Priming with a DNA vector co-administered with gp120 Env protein generated the most rapid, potent and durable binding antibody response among the four vaccine regimens tested.
"DNA-based vaccines may represent an interesting strategy in combination with protein components to induce potent antibody responses," Dr. Pantaleo said. "This vaccine regimen may be instrumental for the development of vaccines against other difficult targets."
Dr. Barbara K. Felber of the National Cancer Institute, in Frederick, Maryland, who co-authored an accompanying editorial, told Reuters Health by email, "This study shows that inclusion of protein in the priming vaccination resulted in high humoral immune responses in humans. This changes the paradigm of using protein only in the booster phase of vaccinations."
"Interestingly, this study confirms what we found in the
macaque model and this is another important finding and validates the
effort for non-human macaque studies," she said. "This study also shows
that inclusion of protein in the prime improves the rapid development of
immune responses using different vaccine platforms (DNA, NYVAC). Thus,
this demonstrates a more general application of improving the priming
vaccination."
"We need to be innovative and explore novel ways to improve vaccine efficacy for HIV and likely other vaccines," Dr. Felber said.
Dr. Felber and co-author Dr. George N. Pavlakis are co-inventors of U.S. government-owned patents for DNA vaccine technology and DNA immunotherapy.
SOURCE: https://bit.ly/2oP0cJ3 and https://bit.ly/2N0WgNm
Lancet HIV 2019.
"If the ongoing vaccine efficacy studies show significant levels of protection against acquisition of HIV, future vaccine studies may be designed to induce rapid appearance of antibody responses as shown in our study," Dr. Giuseppe Pantaleo from the Swiss Vaccine Research Institute, Lausanne University Hospital, told Reuters Health by email.
Dr. Pantaleo and colleagues in the HVTN 096 trial tested their hypothesis that coadministration of vaccines containing Env protein during priming in a heterologous prime and boost regimen would induce early generation of an antibody response against the conserved regions of the V1/V2 loop of Env, thereby extending the period of protection seen in an earlier trial (RV144).
Specifically, they compared vaccine regimens comprising coadministration during priming of either a DNA or NYVAC vector and gp120 Env protein versus DNA or NYVAC vector alone followed by a NYVAC vector and gp120 protein boost.
Vaccines containing the NYVAC vector triggered more-frequent severe reactogenicity and more adverse events than did vaccines containing the DNA vector, the researchers report in The Lancet HIV, online October 7.
Co-administration of gp120 Env protein during priming resulted in earlier peak neutralizing antibody responses, compared with regimens lacking gp120 Env protein at prime.
Gp120 Env protein co-administration during priming also yielded a significantly higher overall neutralizing antibody response (as measured by AUC), compared with groups that did not receive gp120 Env protein co-administration at prime.
For all groups, antibody responses and magnitudes of response decreased between two weeks and 12 months after the month-six vaccination, and there were no differences in the aggregate vaccine-matched response magnitudes between groups at 12 months after the month-six vaccination.
Priming with a DNA vector co-administered with gp120 Env protein generated the most rapid, potent and durable binding antibody response among the four vaccine regimens tested.
"DNA-based vaccines may represent an interesting strategy in combination with protein components to induce potent antibody responses," Dr. Pantaleo said. "This vaccine regimen may be instrumental for the development of vaccines against other difficult targets."
Dr. Barbara K. Felber of the National Cancer Institute, in Frederick, Maryland, who co-authored an accompanying editorial, told Reuters Health by email, "This study shows that inclusion of protein in the priming vaccination resulted in high humoral immune responses in humans. This changes the paradigm of using protein only in the booster phase of vaccinations."
"We need to be innovative and explore novel ways to improve vaccine efficacy for HIV and likely other vaccines," Dr. Felber said.
Dr. Felber and co-author Dr. George N. Pavlakis are co-inventors of U.S. government-owned patents for DNA vaccine technology and DNA immunotherapy.
SOURCE: https://bit.ly/2oP0cJ3 and https://bit.ly/2N0WgNm
Lancet HIV 2019.

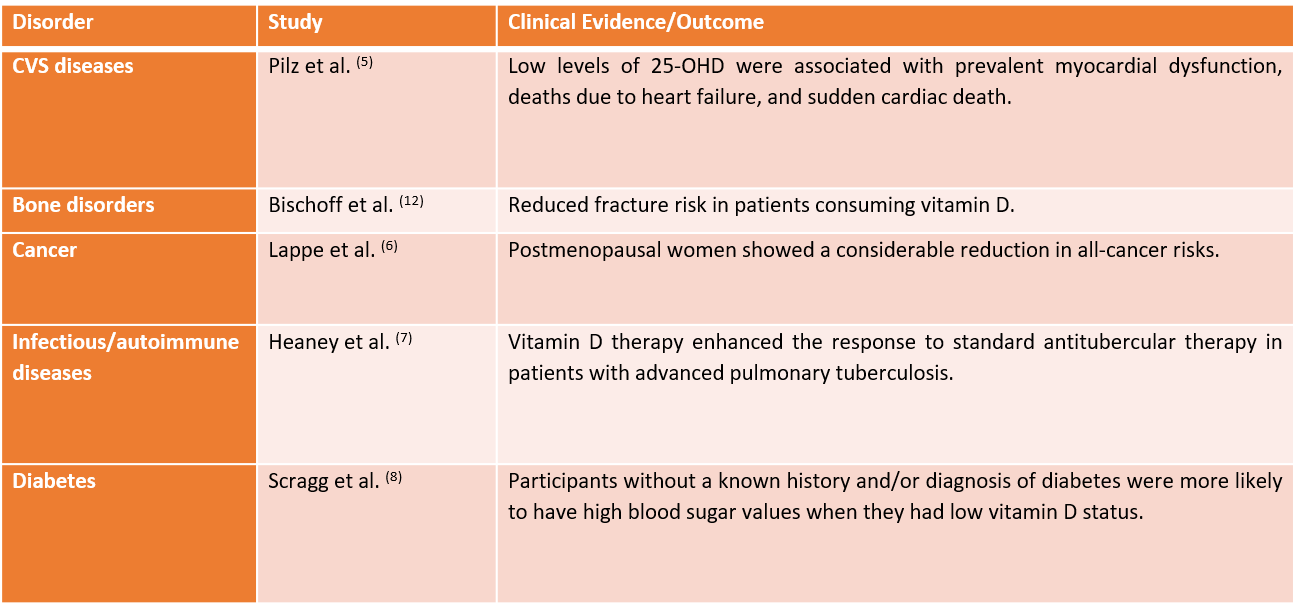
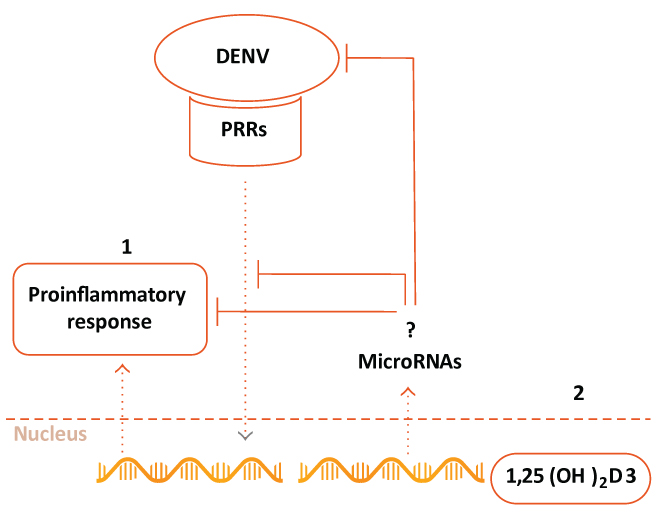
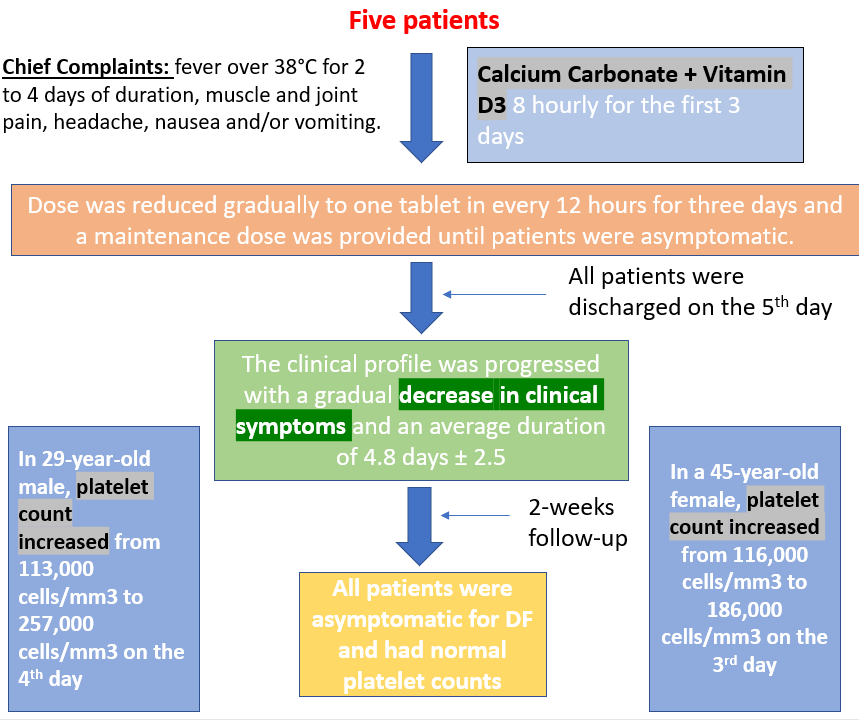
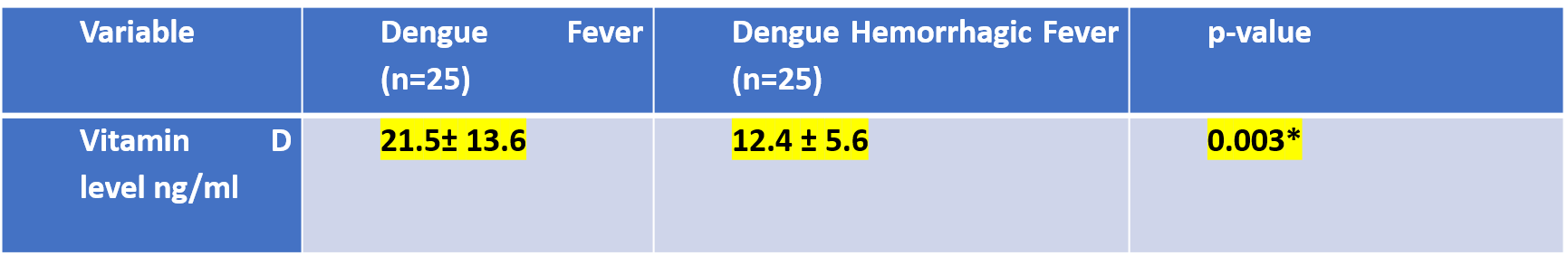 Adapted from Mahmud MR et al. *independent sample t-test; # chi-square test
Adapted from Mahmud MR et al. *independent sample t-test; # chi-square test